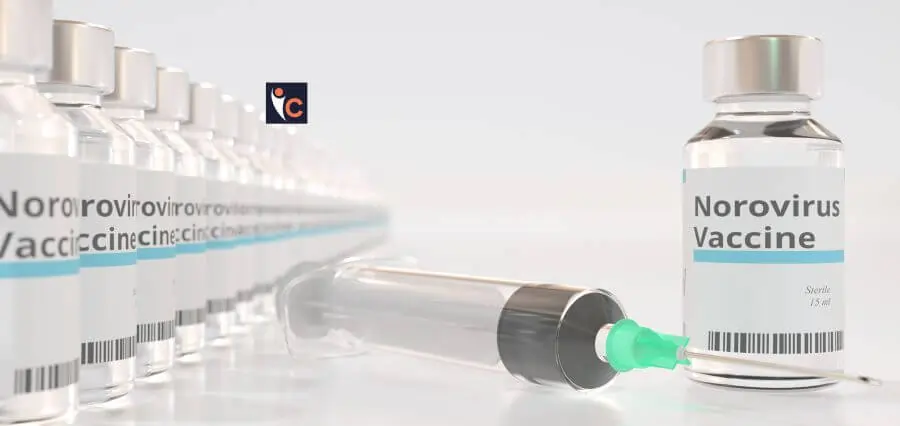
The UK is gearing up to test the world’s first vaccine designed to combat norovirus, also known as the “winter vomiting bug.” This groundbreaking trial could significantly reduce the burden on the NHS and improve public health.
What Is Norovirus?
- Highly Contagious: Norovirus spreads easily, causing vomiting and diarrhea that typically last two to three days.
- Year-Round Impact: Though most common in winter, it affects people of all ages throughout the year.
- Global Burden: Norovirus accounts for approximately 685 million cases worldwide annually, including 4 million in the UK.
Vaccine Details and Technology
- mRNA-Based Vaccine: Moderna’s vaccine uses mRNA technology to help the immune system recognize and attack the virus.
- Targets Three Strains: It focuses on strains responsible for 67% of norovirus cases.
- Phase 3 Trial: The vaccine has shown a strong immune response and will now undergo large-scale testing.
UK’s Role in the Trial
- Nationwide Participation:
- 27 NHS hospitals and health centers across England, Scotland, and Wales are involved.
- Mobile clinics may visit care homes and community sites.
- Recruitment Goals: 2,500 participants, especially those aged 60 and above, are being sought.
Expected Benefits of the Vaccine
- Reduce Health Risks:
- Protect vulnerable groups like older adults and immunocompromised individuals.
- Prevent severe complications that cause up to 80 deaths annually in the UK.
- Ease NHS Strain:
- Norovirus hospital admissions cost the NHS around £100 million each year.
- A successful vaccine could lower these expenses significantly.
- Broader Impact:
- Potential to prevent 65% or more of norovirus cases.
- Could lead to seasonal vaccination programs similar to flu shots or offer lifelong immunity.
Global and Government Collaboration
- International Efforts: The trial involves the US, Canada, Japan, and potentially Australia.
- Strategic Partnership: Moderna and the UK government are collaborating on vaccine research and production under a 10-year plan.
Future Steps and Optimism
- Data Collection: Participants will be monitored for 25 months.
- Expansion to Children: If successful in adults, trials may extend to younger populations.
- Regulatory Approval: Moderna aims to file for vaccine approval by 2026.
Health Secretary Wes Streeting emphasized the importance of innovation:
“A norovirus vaccine could save lives, reduce NHS pressure, and shift healthcare from treatment to prevention during winter months.”
This initiative positions the UK as a global leader in tackling seasonal illnesses through advanced vaccine development.